A baseline that rises or drops when using flash chromatography with a UV detector can be a problem, especially if you are trying to collect compounds with poor detectability or that exist in low quantities.
In this post I will talk about the causes and solutions for a rising (or even dropping) baseline.
A chromatographic baseline that changes during a run can be catastrophic to your results. If the baseline rises it may obscure compound detection or increase fraction collection volume. If the baseline drops, the separated compounds may not be detected. So, why does this happen and what do you do about it?
The most common reason for baseline changes during a gradient run when a UV or UV-vis detector is used is that the mobile phase solvents absorb UV at different wavelengths during the purification run. Figure 1 shows this with a normal-phase purification using hexanes and ethyl acetate solvents. Using a diode-array UV-vis detector and detecting with all available wavelengths (200-800 nm) as shown by the tan-colored trace, the baseline rises notably. This is because ethyl acetate absorbs UV between 200 and 252 nm, Figure 2. The red and black traces are specified wavelengths in the visible spectra range where ethyl acetate is transparent.
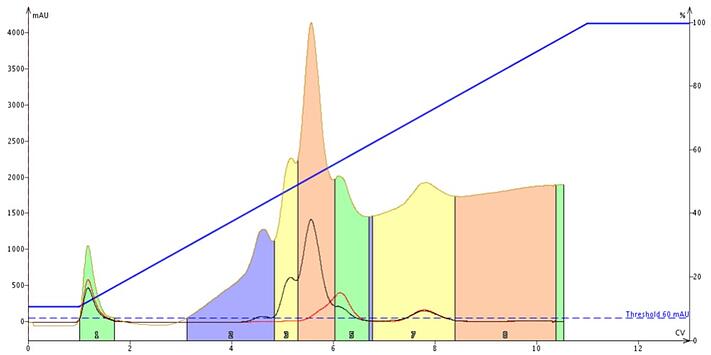 Figure 1. An A to B gradient elution using heptane (solvent A) and ethyl acetate (solvent B). Ethyl acetate absorbs UV light up to about 252 nm. The tan trace shows UV absorption across the full wavelength range, while the black and red traces are specific wavelengths selected for the sample (both are above 252 nm). If the strong solvent (typically solvent B in an A to B gradient) absorbs UV, then the baseline will rise, if the weak solvent absorbs UV as with a DCM/MeOH gradient, then the baseline can drop. For compounds absorbing in this UV range, detection may be compromised.
Figure 1. An A to B gradient elution using heptane (solvent A) and ethyl acetate (solvent B). Ethyl acetate absorbs UV light up to about 252 nm. The tan trace shows UV absorption across the full wavelength range, while the black and red traces are specific wavelengths selected for the sample (both are above 252 nm). If the strong solvent (typically solvent B in an A to B gradient) absorbs UV, then the baseline will rise, if the weak solvent absorbs UV as with a DCM/MeOH gradient, then the baseline can drop. For compounds absorbing in this UV range, detection may be compromised.
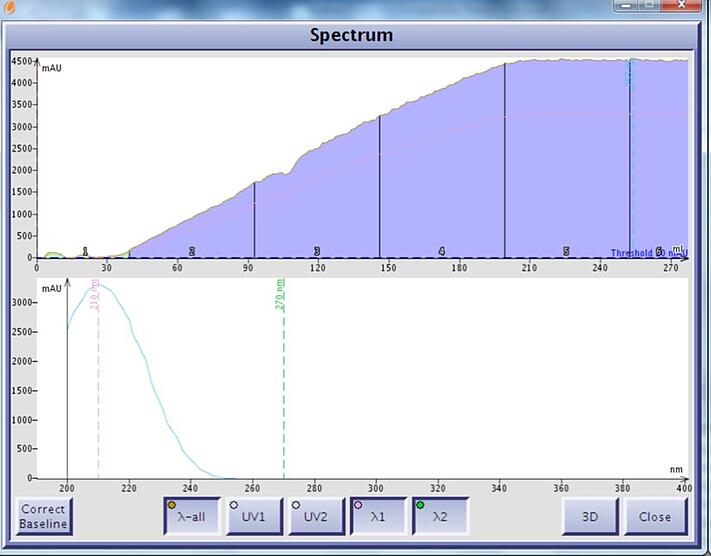 Figure 2. Ethyl acetate UV spectrum and chromatographic UV absorption in a gradient (0-100%) without correction. Ethyl acetate has a UV maximum at 210 nm and a UV cutoff beyond 250 nm.
Figure 2. Ethyl acetate UV spectrum and chromatographic UV absorption in a gradient (0-100%) without correction. Ethyl acetate has a UV maximum at 210 nm and a UV cutoff beyond 250 nm.
Other reasons for changing baselines include...
- Changes in solvent refractive index during gradient elution
- Inadequate cartridge equilibration (not enough equilibration solvent)
- Dirty stationary phase (a good reason not to reuse silica cartridges)
- UV absorbing pH modifiers
So how do we prevent a changing baseline from occurring? Well, as seen in Figure 1, individual wavelengths can be selected which are beyond the UV cutoff. While this will work, detection at specific wavelengths usually reduces the amount of material collected (notice the reduced peak heights for the red and black traces compared to the tan trace in Figure 1). If compound recovery or yield is a chromatographic goal, this may not be the ideal solution.
Other options include...
- -Replacing your used silica column
- -Use the baseline correction capability of your flash system if it has that feature
- -Fully equilibrate your column, either normal- or reversed-phase with at least 2 CV of mobile phase
- -Ensure equal pH modifier concentration in both solvents
- -Clean your used reversed-phase column with increasingly stronger solvents - methanol then acetonitrile then acetone - to remove lipophilic compounds
In my experience, the way to best address this issue is to use fresh cartridges and have the flash system correct itself in real time for the solvents' UV absorption. Unfortunately, not all automated flash systems have this capability, but if yours does, take advantage of it.
Baseline correction, at least with the Biotage® Spektra, eliminates a changing baseline in real time, Figure 3. By subtracting the solvent's UV absorption during the purification run, compounds are more easily detected with better sensitivity and they are collected in much smaller volumes compared to non-corrected baselines (compare to Figure 1).
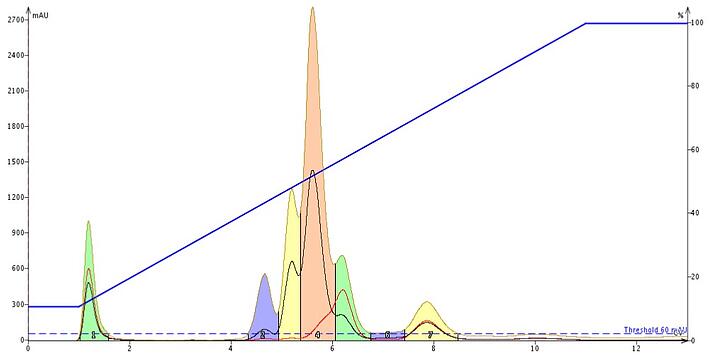 Figure 3. The impact of dynamic, real time baseline correction is seen with this sample. Sensitivity is improved and fraction volumes are reduced compared to a non-corrected baseline.
Figure 3. The impact of dynamic, real time baseline correction is seen with this sample. Sensitivity is improved and fraction volumes are reduced compared to a non-corrected baseline.
Interested in learning more about flash chromatography? Please download our whitepaper - Successful Flash Chromatography.

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership