Purification by reversed-phase chromatography relies primarily on a hydrophobic interaction of the molecule with the alkyl chains bonded to the stationary phase for column retention and elution through a partitioning mechanism. While this is certainly true for purification of peptides, surface area accessibility and media particle size also play critical roles in the resolving power of a particular stationary phase. The particle size influences the loading capacity, however pore size greatly influences molecular accessibility and therefore resolving power.
In today’s post, I will demonstrate how pore size can impact your peptide purification using flash column chromatography.
The influence of pore size is well know in the HPLC column community, as demonstrated by the vast array of columns available that vary in both particle size and pore size, but is not commonly discussed in the flash column chromatography community. Recently, I was asked to evaluate some new C18-functionalized media that has a pore size of approximately 300Å and I jumped at the opportunity to identify the differences.
For this evaluation, I compared the resolving power of the Biotage® SNAP Ultra C18 (nominal 100Å pore) to that of the new Biotage® SNAP Bio C18 (nominal 300Å pore). For this comparison, I felt it critical to use crude peptide from a single synthesis and fortunately I had recently completed a large scale synthesis of ACP(65-74). After conducting analytical RP-HPLC-MS, I calculated the crude sample to be about 36% pure; this sample contains a high concentration of residual protecting groups but importantly, it also includes a des-Val deletion impurity, Figure 1.
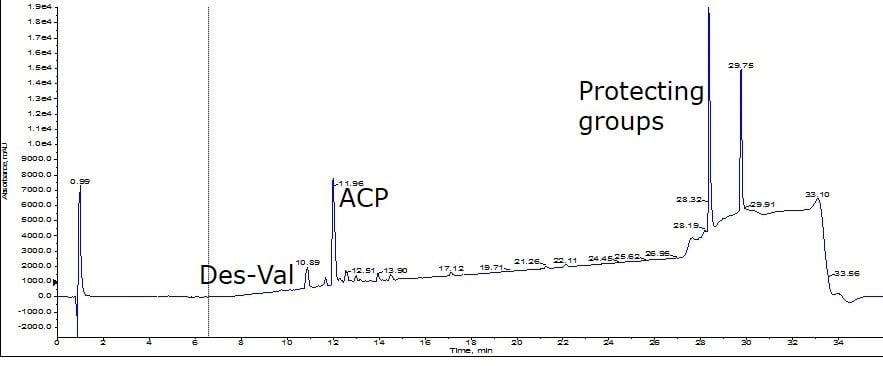
Figure 1: Crude analytical HPLC chromatogram for peptides purified below.
I first loaded about 50 mg of my peptide dissolved in DMSO onto the Biotage® SNAP Ultra C18 12 gram cartridge for purification, Figure 2.
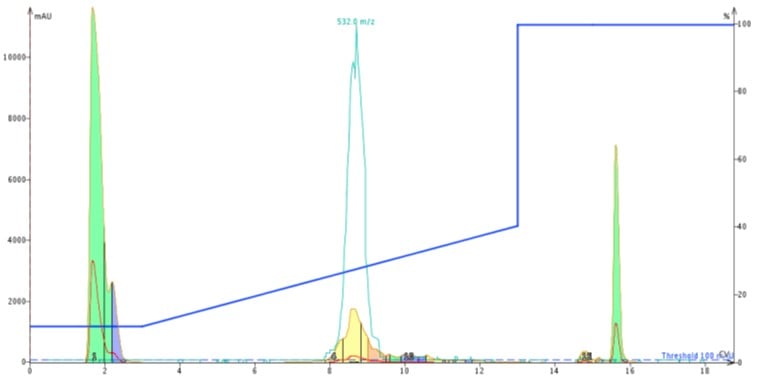
Figure 2: Chromatogram resulting from 50 mg ACP in 300 uL DMSO loaded onto a 12 gram Biotage® SNAP Ultra C18 cartridge. The peptide is purified using a gradient that runs from 10% to 40% acetonitrile in water, both modified with TFA.
The peak shape is certainly not gaussian, suggesting the presence of co-eluting molecules. I monitored the elution by MS, but collected the peaks based on UV absorbance. With these strategies as a guide, I combined fractions 4-6 (center yellow peak), concentrated them with the Biotage® V-10 Evaporation system and re-injected the peptide on my analytical HPLC-MS where I found the sample to be about 77% pure, Figure 3. Critically, the des-Val deletion product is not removed to any measurable extent. Not the best results…
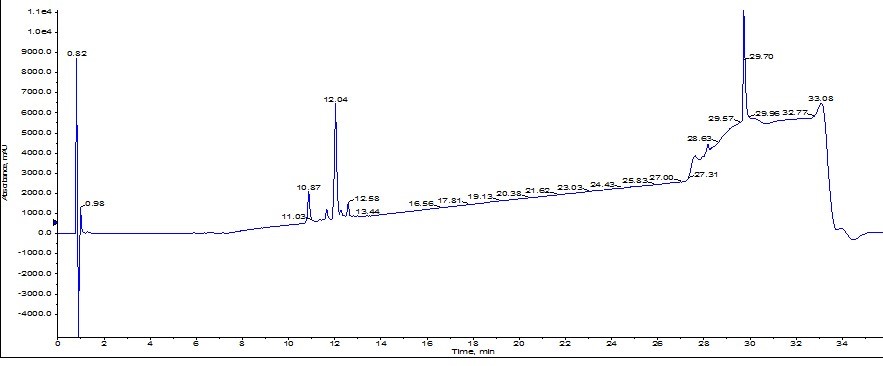
Figure 3: Analytical HPLC of ACP peptide purified using the Biotage® SNAP Ultra C18 cartridge.
For comparison sake, I injected approximately 50 mg of crude ACP dissolved in DMSO onto a Biotage® SNAP Bio C18 cartridge using the exact same gradient as the previous injection, Figure 4.
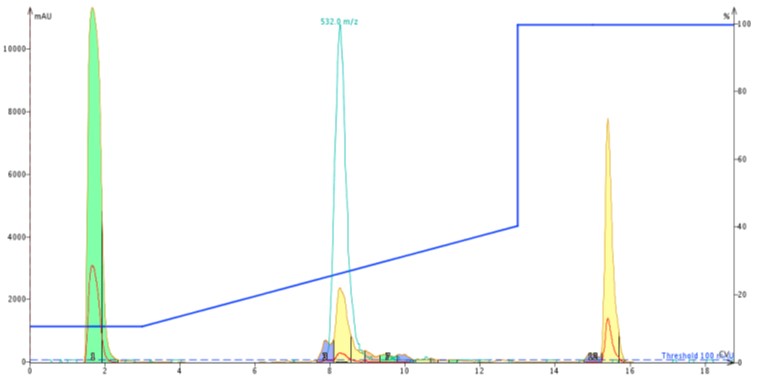
Figure 4: Chromatography of 50 mg crude ACP loaded on a Biotage® SNAP Bio C18 cartridge.
Results from the larger pore media show a distinct leading peak which was sufficiently resolved to trigger a fraction change. Using the MS and UV traces as a guide, I combined fractions 4 and 5 (middle yellow peak), quickly concentrated them with the Biotage V-10 evaporation system and reinjected onto the analytical HPLC-MS as before. Using the analytical HPLC-MS, I calculated the sample to be approximately 92% pure, Figure 5. A significant improvement over the previous sample!
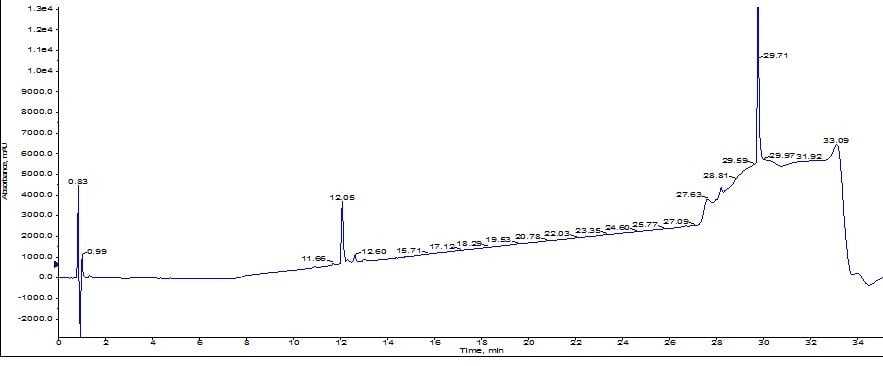
Figure 5: Analytical HPLC chromatogram for ACP purified using the Biotage® SNAP Bio C18 cartridge.
As a follow up experiment, I decided to reinject the peptide originally purified using the Biotage® SNAP Ultra C18 onto the new Biotage® SNAP Bio C18. The second chromatogram clearly shows the expected leading peak, which is still resolved sufficiently to trigger fractionation, even at significantly lower loading levels, Figure 6.
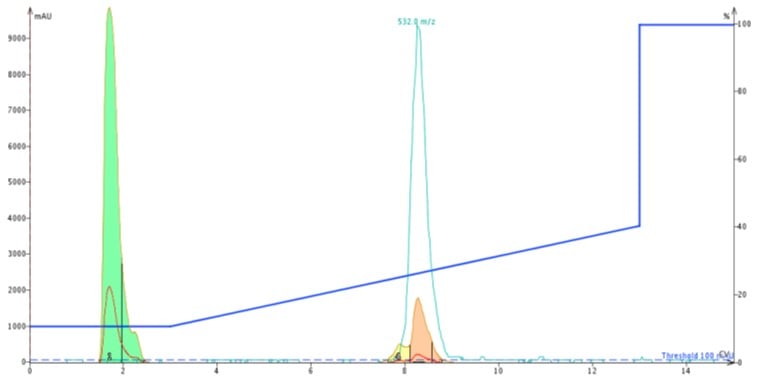
Figure 6: Chromatogram for second purification of ACP using the Biotage SNAP Bio C18 cartridge.
Analysis of the resulting analytical HPLC clearly indicate that the second purification effort did in fact improve the overall purity (up to 93% from the original 77% purity), Figure 7.
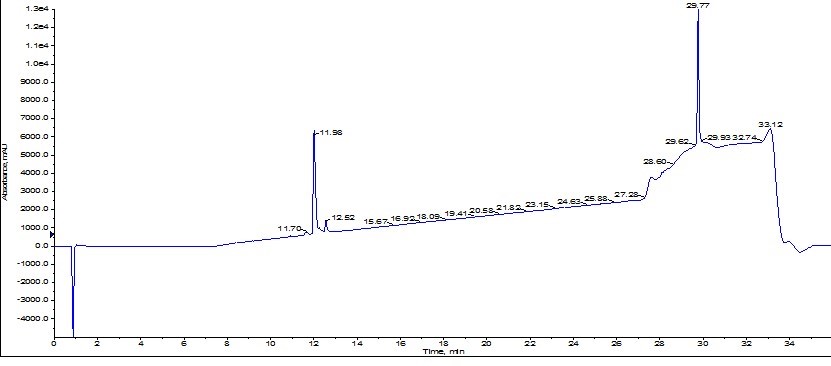
Figure 7: Analytical HPLC chromatogram resulting from a second purification using the Biotage® SNAP Bio C18 of ACP originally purified using the Biotage® SNAP Ultra C18 cartridge.
The dramatic increase in purity resulting from the purification attempt suggests that the improvement in purification is due to the increased particle pore size, rather than an artifact result from amount of material loaded on the column. The larger pore size enables greater molecular accessibility to the stationary phase alkyl chains inside the pores, thereby increasing the probability that the molecule of interest will have maximal interaction with the stationary phase. For flash chromatography, run with low back pressures, it is clear that the larger pore size yields a higher purity peptide product in a single injection. I expect that this will also follow for larger peptides as well, but that’s for a later discussion.
To learn other strategies that enable recovery of highly pure peptide using flash chromatography, follow the link below.
Learn More about High Purity Peptides

 Organic Workflow
Organic Workflow Peptide Workflow
Peptide Workflow Scale-Up Flash Purification
Scale-Up Flash Purification  Sample Preparation
Sample Preparation Biomolecule Purification
Biomolecule Purification Oligo synthesis
Oligo synthesis Scavengers and Reagents
Scavengers and Reagents Service & Support
Service & Support Accessories & Spare parts
Accessories & Spare parts Investors
Investors Reports & News
Reports & News The Share
The Share Corporate Governance
Corporate Governance Calendar
Calendar Sustainability
Sustainability Our Offering
Our Offering Our History
Our History Our Locations
Our Locations Leadership
Leadership